
Listen To This Blog
Introduction: Unveiling the Essence of Physical Chemistry
In the realm of scientific exploration, physical chemistry stands as a pivotal discipline, merging the meticulousness of physics with the diversity of chemistry. This field is not just about atoms and molecules; it's an intricate dance of energy and matter, revealing the profound secrets of the universe at both the macroscopic and subatomic levels. Let's delve into the core aspects of physical chemistry, uncovering its significance and the various branches that define this fascinating area of study.
- The Atomic and Molecular Symphony: At its heart, physical chemistry examines how atoms and molecules interact, bond, and transform, offering a window into the fundamental principles that govern our world.
- Energy, Forces, and Reactions: The study extends beyond mere composition, delving into the dynamics of energy, the forces at play, and the reactions that reshape matter in myriad ways.
- Bridging Concepts and Real-World Applications: Physical chemistry is not confined to theoretical constructs; it finds practical applications in everyday phenomena and technological advancements, influencing various scientific and industrial fields.
Embarking on this journey through physical chemistry, we will explore its diverse branches, each contributing a unique perspective to our understanding of the natural world. From the heat and work interplay in thermodynamics to the electron's journey in electrochemistry, each branch offers a piece of the puzzle in our quest to comprehend the universe's intricate workings.
Understanding Chemistry
Chemistry is a fascinating and diverse field of science that plays a crucial role in our understanding of the world. It is the study of matter, its properties, how and why substances combine or separate to form other substances, and how these substances interact with energy.
- The Essence of Matter: At its core, chemistry examines the composition, structure, and properties of matter. It delves into the intricacies of atoms and molecules, the basic units of matter, revealing how they bond and react.
- Chemical Reactions and Energy: A significant aspect of chemistry is understanding chemical reactions. It explores how substances change and release or absorb energy, providing insights into various processes from biological functions to industrial applications.
- Organic and Inorganic Realms: Chemistry branches into organic, studying carbon-containing compounds, and inorganic chemistry, focusing on non-organic compounds. This division highlights the vast scope and applicability of chemistry in different domains.
In essence, chemistry is not just a subject; it's a window to understanding the fundamental processes of life and the universe.
Exploring the Diverse Branches of Chemistry
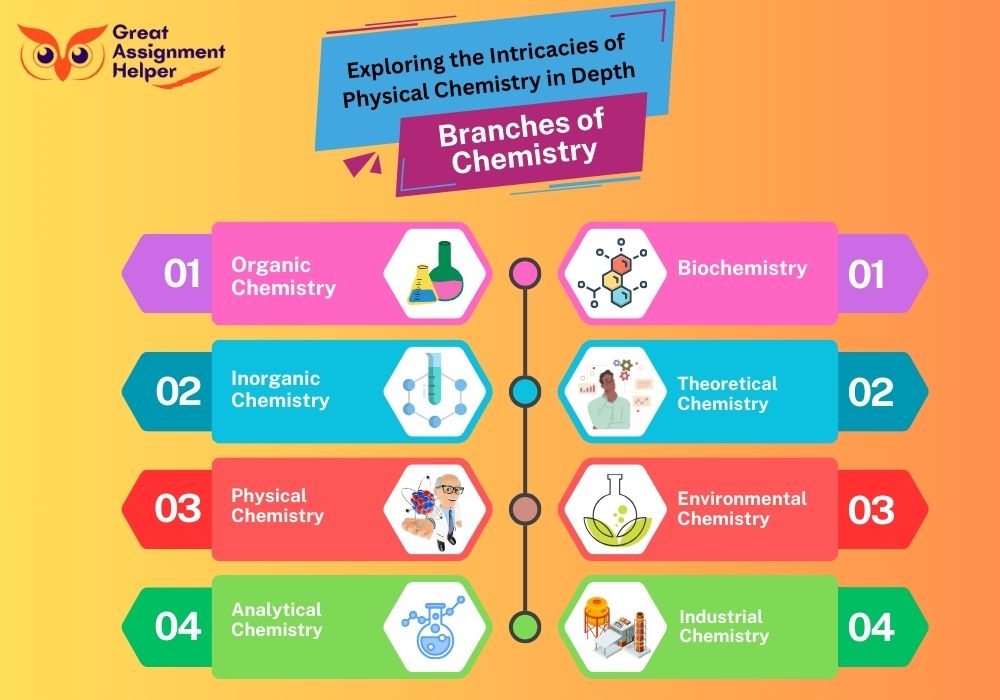
Chemistry, a multifaceted science, branches out into several specialized fields, each focusing on different aspects of matter and its interactions. Understanding these branches provides a comprehensive view of the subject's breadth and depth.
- Analytical Chemistry: This branch is centered on analyzing the composition of materials. It involves techniques and methods to identify, quantify, and separate substances in samples, playing a crucial role in quality control and assurance.
- Physical Chemistry: Bridging chemistry with physics, this field studies how matter behaves on a molecular and atomic level. It delves into the principles of thermodynamics, kinetics, and quantum mechanics, providing a deeper understanding of chemical reactions and properties.
- Organic Chemistry: Focused on carbon-containing compounds, organic chemistry explores the structure, properties, synthesis, and reactions of these molecules. It's fundamental in pharmaceuticals, plastics, and many other industries.
- Inorganic Chemistry: This branch deals with inorganic compounds, primarily those without carbon. It covers a wide range of substances, from minerals and metals to semiconductors, influencing material science and catalysis.
- Biochemistry: At the intersection of chemistry and biology, biochemistry examines the chemical processes within and related to living organisms. It's crucial for understanding biological functions and disease mechanisms.
Each branch of chemistry, with its unique focus and methodologies, contributes significantly to our understanding of the natural world and the development of new technologies and solutions.
Unraveling the Mysteries of Physical Chemistry
Physical chemistry stands at the crossroads of chemistry and physics, delving into the physical principles that govern the behavior and properties of chemical systems. This fascinating field combines theoretical insights and experimental techniques to understand the fundamental aspects of matter and energy.
Core Concepts and Theories:
At its heart, physical chemistry seeks to comprehend the principles that underlie chemical phenomena. This involves exploring atomic and molecular structures, chemical reactions, and the properties of materials.
- Quantum Mechanics: A pivotal part of physical chemistry, quantum mechanics, provides a theoretical framework for understanding the electronic structure of atoms and molecules. It explains how these entities interact and bond, forming the basis for chemical reactions.
- Thermodynamics: This area focuses on the study of energy, heat, work, and their transformations in chemical processes. It helps in understanding the spontaneity of reactions and the equilibrium states of chemical systems.
Kinetics and Reaction Dynamics:
Physical chemistry also investigates the speed and pathways of chemical reactions. Kinetics, a key component, examines the rate at which reactions occur and the factors influencing these rates.
- Catalysis: Understanding how catalysts speed up reactions without being consumed is a crucial aspect of kinetics. This knowledge is vital for developing efficient industrial processes and environmentally friendly technologies.
Spectroscopy and Molecular Structure:
Spectroscopy, an essential tool in physical chemistry, involves studying the interaction of electromagnetic radiation with matter. It provides insights into the structure and dynamics of molecules.
- NMR and IR Spectroscopy: Techniques like Nuclear Magnetic Resonance (NMR) and Infrared (IR) Spectroscopy are instrumental in elucidating molecular structures and understanding molecular behavior.
Materials Chemistry and Nanotechnology:
Physical chemistry plays a significant role in the development of new materials and nanotechnology. It helps in designing and characterizing materials with novel properties for various applications.
- Semiconductors and Superconductors: The study of these materials has led to advancements in electronics and quantum computing, showcasing the practical impact of physical chemistry.
Surface Chemistry and Electrochemistry:
These subfields explore the chemical processes at surfaces and interfaces, and the flow of electric charge in chemical reactions, respectively. They are fundamental in understanding corrosion, battery technology, and electroplating.
In summary, physical chemistry is a dynamic and integral part of the scientific world, offering profound insights into the molecular and atomic levels of matter. Its principles and methodologies are essential in various fields, from pharmaceuticals to renewable energy, making it a cornerstone of modern scientific research and application.
Branches of Physical Chemistry
Physical chemistry, a significant branch of chemistry, encompasses various sub-disciplines, each focusing on specific aspects of the physical properties and transformations of materials. Below is a table outlining these branches:
|
Branch |
Description |
|---|---|
|
Thermodynamics |
Focuses on the study of energy changes and transfers in chemical reactions, including concepts like enthalpy, entropy, and free energy. |
|
Quantum Chemistry |
Deals with the application of quantum mechanics to chemical systems, explaining the behavior of electrons in atoms and molecules. |
|
Chemical Kinetics |
Studies the rate at which chemical reactions occur and the factors that influence these rates, such as temperature and catalysts. |
|
Spectroscopy |
Involves the interaction of electromagnetic radiation with matter to analyze and identify chemical substances. |
|
Electrochemistry |
Examines the relationship between electricity and chemical change, including the study of electrochemical cells and reactions. |
|
Photochemistry |
Focuses on the chemical reactions that occur as a result of light absorption, crucial in processes like photosynthesis. |
|
Statistical Mechanics |
Applies the laws of statistics to predict the properties of a system from the microscopic behavior of its constituents. |
|
Surface Chemistry |
Investigates the processes occurring at surfaces and interfaces, essential in understanding catalysis and adsorption. |
|
Materials Chemistry |
Explores the physical properties of materials, particularly solids, and their application in various technologies. |
|
Biophysical Chemistry |
Combines the principles of physical chemistry with biological systems to understand biochemical processes at a molecular level. |
Each of these branches plays a vital role in expanding our understanding of the physical aspects of chemical substances and their transformations, contributing significantly to advancements in science and technology.
Understanding Thermodynamics
Thermodynamics is a fundamental branch of physics and chemistry, focusing on the study of heat, energy, and their transformations. It's a field that intersects various scientific disciplines, offering insights into how energy is transferred and converted in different systems.
- Core Concepts: At the heart of thermodynamics lie several key concepts, including energy, heat, work, and entropy. These principles form the basis for understanding how energy moves and changes form, influencing everything from microscopic particles to vast systems.
- Laws of Thermodynamics: The framework of thermodynamics is built on four main laws. The Zeroth Law establishes the concept of temperature. The First Law, also known as the law of energy conservation, states that energy cannot be created or destroyed, only transformed. The Second Law introduces the concept of entropy, indicating that systems tend to move towards disorder. The Third Law suggests that as temperature approaches absolute zero, the entropy of a system approaches a constant minimum.
- Applications and Implications: Thermodynamics has wide-ranging applications, from engineering and environmental science to biology and cosmology. It helps in understanding the efficiency of engines, the behavior of materials under different temperatures, and the physical limits of energy conversion.
In summary, thermodynamics provides a crucial framework for understanding the fundamental principles governing energy and its transformations, playing a vital role in both theoretical and applied sciences.
Understanding Chemical Kinetics
Chemical kinetics is a vital branch of chemistry that focuses on the rate at which chemical reactions occur and the factors influencing these rates. It's a field that combines theoretical analysis with practical experiments to understand reaction mechanisms.
- Rate of Reaction: This is the core focus of chemical kinetics. It examines how quickly reactants transform into products, providing insights into the efficiency and feasibility of chemical processes.
- Factors Affecting Reaction Rates: Several factors influence the speed of chemical reactions. These include the concentration of reactants, temperature, presence of catalysts, and the physical state of the reactants. Each factor plays a crucial role in determining how fast a reaction proceeds.
- Reaction Mechanisms: Kinetics also delves into the step-by-step sequence of events at the molecular level during a reaction. Understanding these mechanisms helps in predicting the course of reactions and designing new ones for various applications.
- Mathematical Modeling: The field employs mathematical equations to describe and predict the kinetics of chemical reactions. These models are essential for theoretical predictions and practical applications in industries and research.
Chemical kinetics, with its blend of theoretical and practical aspects, is essential for advancing our understanding of chemical processes, from biological systems to industrial manufacturing.
Unveiling the World of Photochemistry
Photochemistry is a captivating branch of chemistry that focuses on the chemical reactions, changes, and processes that result from the absorption of light. This field is integral to understanding numerous natural and artificial phenomena.
- Light-Induced Chemical Reactions: At the heart of photochemistry is the study of how light interacts with molecules, leading to chemical changes. This includes understanding how photons (light particles) initiate and influence reactions at the molecular level.
- Photosynthesis and Natural Processes: A key example of photochemistry in nature is photosynthesis, where plants convert light energy into chemical energy. This process is fundamental to life on Earth, showcasing the vital role of photochemistry in natural systems.
- Technological Applications: Photochemistry has practical applications in various fields, including the development of solar energy, phototherapy in medicine, and the creation of light-sensitive materials. Its principles are also applied in photography and printing technologies.
- Environmental Impact: Understanding photochemical reactions is crucial in assessing environmental factors like the formation of smog and the depletion of the ozone layer. It helps in developing strategies to mitigate negative environmental impacts.
Photochemistry, thus, represents a dynamic intersection of chemistry and physics, offering insights into both fundamental scientific principles and practical applications in our daily lives.
Quantum Chemistry: A Modern Perspective
Quantum Chemistry stands at the crossroads of chemistry and quantum physics, offering profound insights into molecular structures and reactions. This field is pivotal in understanding the physical basis of chemical phenomena.
- Fundamental Principles: At its heart, Quantum Chemistry applies the principles of quantum mechanics to chemical systems. It explains how electrons and nuclei behave in atoms and molecules, providing a theoretical framework for understanding chemical bonding and electronic properties.
- Molecular Modeling: A key application of Quantum Chemistry is in molecular modeling. This involves using computational methods to predict the structures, reactivities, and properties of molecules, which is invaluable in drug design and materials science.
- Spectroscopy and Reactions: Quantum Chemistry also plays a critical role in interpreting spectroscopic data, helping scientists understand how molecules absorb and emit light. Additionally, it sheds light on the mechanisms of chemical reactions at the quantum level.
- Advancements in Technology: With the advent of powerful computers, Quantum Chemistry has grown rapidly, enabling more accurate and complex simulations. This progress is driving innovations in various fields, from nanotechnology to renewable energy.
Quantum Chemistry, by merging quantum physics with chemical intuition, provides a deeper understanding of the microscopic world, opening new frontiers in scientific research and technological development.
Electrochemistry: The Intersection of Electricity and Chemical Reactions
Electrochemistry is a fascinating branch of chemistry that studies the relationship between electrical energy and chemical changes. This field is pivotal in various applications, from battery technology to electroplating.
- Fundamentals of Electrochemical Reactions: At the heart of electrochemistry is the study of redox (reduction-oxidation) reactions, where the transfer of electrons occurs between substances. These reactions are fundamental to generating electrical energy or using electrical energy to drive chemical changes.
- Batteries and Energy Storage: A key application of electrochemistry is in the development of batteries. By harnessing redox reactions, batteries store chemical energy and convert it into electrical energy, playing a crucial role in powering a wide range of devices.
- Electroplating and Corrosion: Electrochemistry also explores electroplating, where a metal is deposited onto a surface using an electric current. This process is essential in manufacturing, jewelry making, and preventing corrosion in metals.
- Environmental Applications: Electrochemical methods are increasingly used in environmental applications, such as water purification and the development of renewable energy sources, highlighting the field's growing importance in sustainable technologies.
Electrochemistry, by bridging chemical reactions and electrical energy, opens up a world of possibilities in both scientific understanding and practical applications.
Material Chemistry: A Closer Look
Material Chemistry is a dynamic and innovative field that focuses on the development and characterization of new materials.
- Synthesis and Design: It involves creating substances with specific properties and functions, tailored for various applications from electronics to biomedicine.
- Analysis and Properties: This branch extensively studies the physical and chemical properties of materials, determining their suitability for specific uses.
- Innovation in Applications: Material Chemistry is pivotal in advancing technology, contributing to breakthroughs in areas like renewable energy, nanotechnology, and smart materials.
In essence, Material Chemistry is at the forefront of scientific progress, blending creativity with practicality to shape the future.
Biophysical Chemistry: An Interdisciplinary Science
Biophysical chemistry stands at the crossroads of biology, physics, and chemistry, offering a unique perspective on the molecular mechanisms of life.
- Molecular Dynamics: This field delves into the physical properties of biological molecules, exploring how they move, interact, and change shape within cells.
- Energy and Life Processes: Biophysical chemistry examines how energy is utilized and transformed in biological systems, shedding light on processes like photosynthesis and cellular respiration.
- Technological Integration: Utilizing advanced technologies, it investigates the structure and function of biomolecules, contributing to advancements in drug design and medical diagnostics.
Biophysical chemistry is pivotal in unraveling the complexities of biological systems, blending physical principles with chemical processes to understand life at a molecular level.
Material Science: A Comprehensive Overview
Material science stands at the forefront of technological innovation, blending various scientific disciplines to enhance our understanding and application of materials.
- Fundamental Understanding: It delves into the properties and behaviors of materials, both natural and synthetic, exploring how they can be manipulated and improved for specific uses.
- Technological Applications: This field plays a pivotal role in developing new materials for various industries, from electronics to aerospace, offering solutions to engineering challenges.
- Interdisciplinary Nature: Material science intersects with physics, chemistry, and engineering, showcasing its broad scope and impact in advancing modern technology and improving everyday life.
Spectroscopy: A Glimpse into Molecular Interactions
Spectroscopy is a pivotal analytical technique in chemistry that involves the study of the interaction between matter and electromagnetic radiation.
- Fundamental Principles: It is based on the concept that each element or compound absorbs and emits light uniquely, allowing for precise identification and analysis.
- Diverse Applications: From determining the structure of complex molecules in organic chemistry to analyzing celestial bodies in astrophysics, spectroscopy has a wide range of applications.
- Technological Advancements: Modern spectroscopy utilizes advanced technologies for more accurate and detailed analysis, contributing significantly to research and development in various scientific fields.
Physical Organic Chemistry: A Closer Look
Physical organic chemistry is a specialized field that merges aspects of physical chemistry and organic chemistry. This branch focuses on understanding the underlying physical principles governing organic reactions.
- Molecular Behavior: It studies how molecular structure influences reactivity and mechanisms in organic compounds, providing insights into reaction pathways.
- Reaction Dynamics: This area delves into the rates of organic reactions, exploring how different conditions and structures affect the speed and outcomes of these reactions.
Physical organic chemistry plays a pivotal role in developing new synthetic methodologies and understanding biological processes at a molecular level.
Conclusion
In summary, the expansive world of chemistry encompasses various branches, each offering unique insights into the fabric of our universe. For students and professionals delving into this complex subject, understanding these diverse areas can be both challenging and rewarding. For those seeking assistance in mastering these concepts, GreatAssignmentHelper stands out as a reliable service provider for chemistry assignment help. Their expertise and resources offer invaluable support, ensuring a deeper comprehension and appreciation of chemistry in all its forms. Whether it's analytical, organic, or biochemistry, greatassignmenthelper is equipped to guide learners through their academic journey in chemistry.
Read More Blogs: Wavelength Formula

